Solving a biological puzzle: why some genes never change
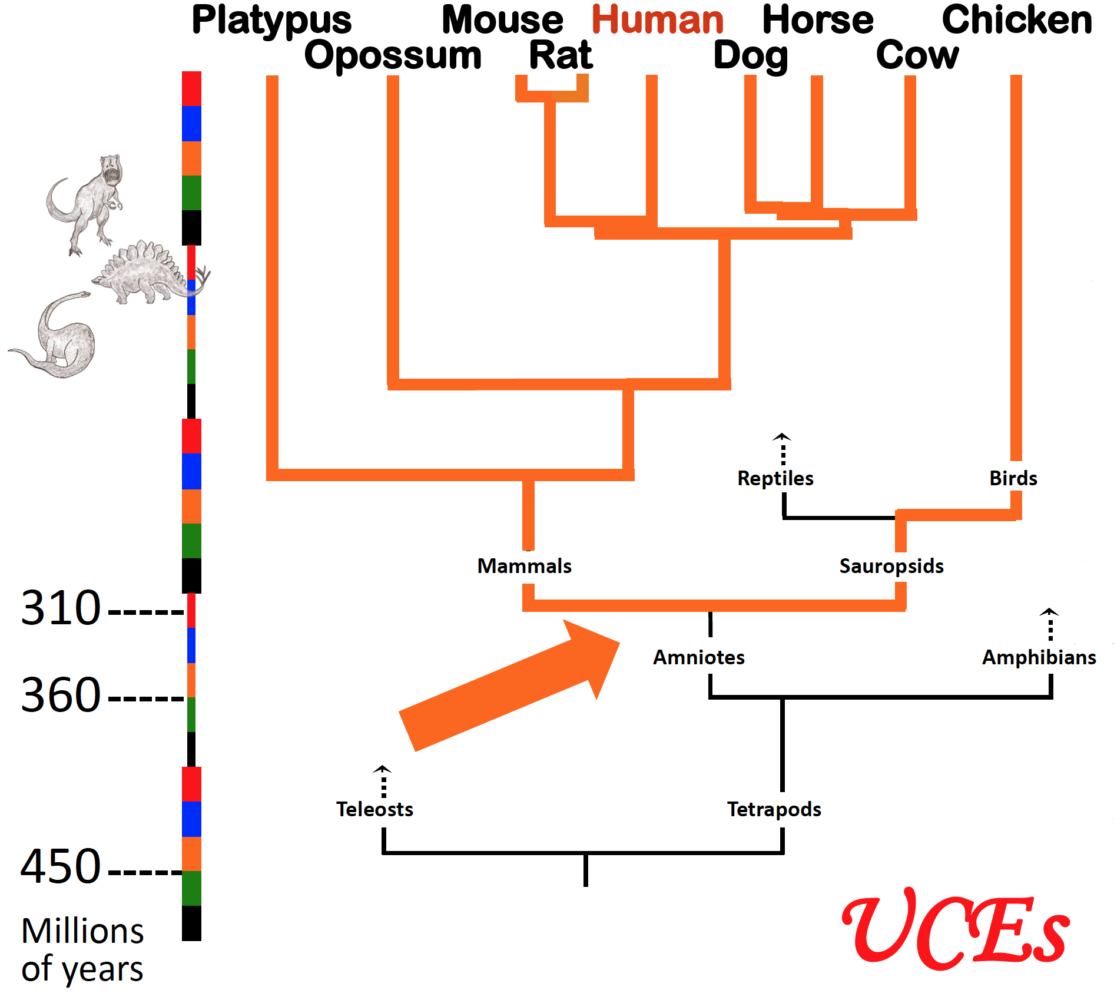
Arrow indicates the moment in evolutionary time when dinosaurs and mammals diverged; both lineages thereafter retained essentially the same ultraconserved elements or UCEs in their genomes. (Graphic courtesy of Ting Wu.)
A mysterious discovery has stumped scientists who study genetics at the cellular level for over a decade. Our genome, or collection of genes, has undergone many evolutionary changes since humankind first emerged millions of years ago, including parts of it that play a critical role in development and survival. Yet hundreds of small segments of our DNA have remained virtually unchanged not only among human beings, but across many other animal species whose lineages diverged before the time of the dinosaurs.
No one yet has convincingly explained how or why.
Ting Wu, a genetics professor at Harvard Medical School, is leading a team of researchers to solve the mystery of these stunningly stable chunks of DNA, called ultraconserved elements or UCEs. The reward for her work may merely be some sort of obscure genetic curiosity. Or it could lead to deep insights into evolution and perhaps reveal mechanisms by which cancer arises and spreads in people and many other creatures. Treatments could follow.
The unsolved mystery
UCEs are exactly the same today as they were 300 million years ago, right down to their individual molecules. Species that evolved from a common ancestor, but are now merely distant relatives, share hundreds of copies of identical UCEs among their genomes.
“It’s really kind of amazing to me,” Wu told an audience of science writers gathered in Cambridge, Mass. Oct. 11, for CASW’s New Horizons in Science briefings at the ScienceWriters2015 conference.
Many scientists believe that UCEs remained unchanged for so long because they must have life-sustaining functions. When other slow-evolving stretches of DNA undergo change, a species will become unfit and die out. The less a gene changes, the idea goes, the more important it is that it stay the same. Wu does not believe this assumption holds up with UCEs. They have no apparent function of their own; they do not order production of some key protein or other metabolic factor. In fact, after scientists completely removed UCEs from mice, they were astonished to find that their cells did not react. The mice remained perfectly healthy and fertile.
Detectives on the case
In her talk on the campus of the Massachusetts Institute of Technology, Wu outlined an unconventional hypothesis that would explain the UCE mystery. She calls the genome’s scattered UCEs its “surveillance system.” Human genes come in two copies, one from our mother and one from our father. Wu believes the maternal and paternal copies of UCEs are somehow screened to make sure they have remained identical as cells have divided and multiplied. If they fail to match up, or there are other errors such as too many or too few copies of a UCE, this may wave a red flag, indicating that the cell has serious damage. If there are enough such signals, her research team proposes, the cell is somehow killed off or ordered to destroy itself. So, for not only humans but all mammals, birds, and reptiles, these ancient, seemingly useless chunks of DNA may in fact play a key role in keeping us healthy. UCEs may effectively scan individual cells for mistakes that could mean the whole genome is in trouble if they persist.
In support of the surveillance hypothesis, Wu and her colleagues found that UCEs behave differently in cancer cells than in normal cells. Unlike healthy cells, which seem to avoid changes to UCEs, cancer cells do contain abnormal numbers of copies of UCEs. Wu believes this reflects a failure of the UCE surveillance system. That in turn might allow cancerous cells to evade whatever it is that UCE errors do to ring the alarm and weed out cells with serious genome problems. Instead, the cancer grows.
Wu admits that UCEs are still largely a mystery, but she says her theoretical supposition is the only one that that fundamentally fits the UCE phenomenon. Her team is now working on new ways to visualize chromosomes in fine detail in the hope of uncovering structural elements of UCEs that bear further clues to their function.
As do all scientists working at the frontier of knowledge, Wu knows that she could be up a blind alley. Much of her lab’s UCE research has not been fully published in scientific journals or other reports. But if the hypothesis holds true, Wu’s research will have implications not so much for treating disease as for preventing it. Wu says harnessing the UCEs’ ability to trigger the death of damaged cells might offer a way to stop a disease “right at the starting point, instead of letting the disease develop and then trying to contain it.” She dreams that her research will lead to a “UCE pill” that would allow people to rid their bodies of diseased cells before they become a problem.
“We’re a long way off,” said Wu, “whether we get there, I have no idea, but it does fuel my research.”